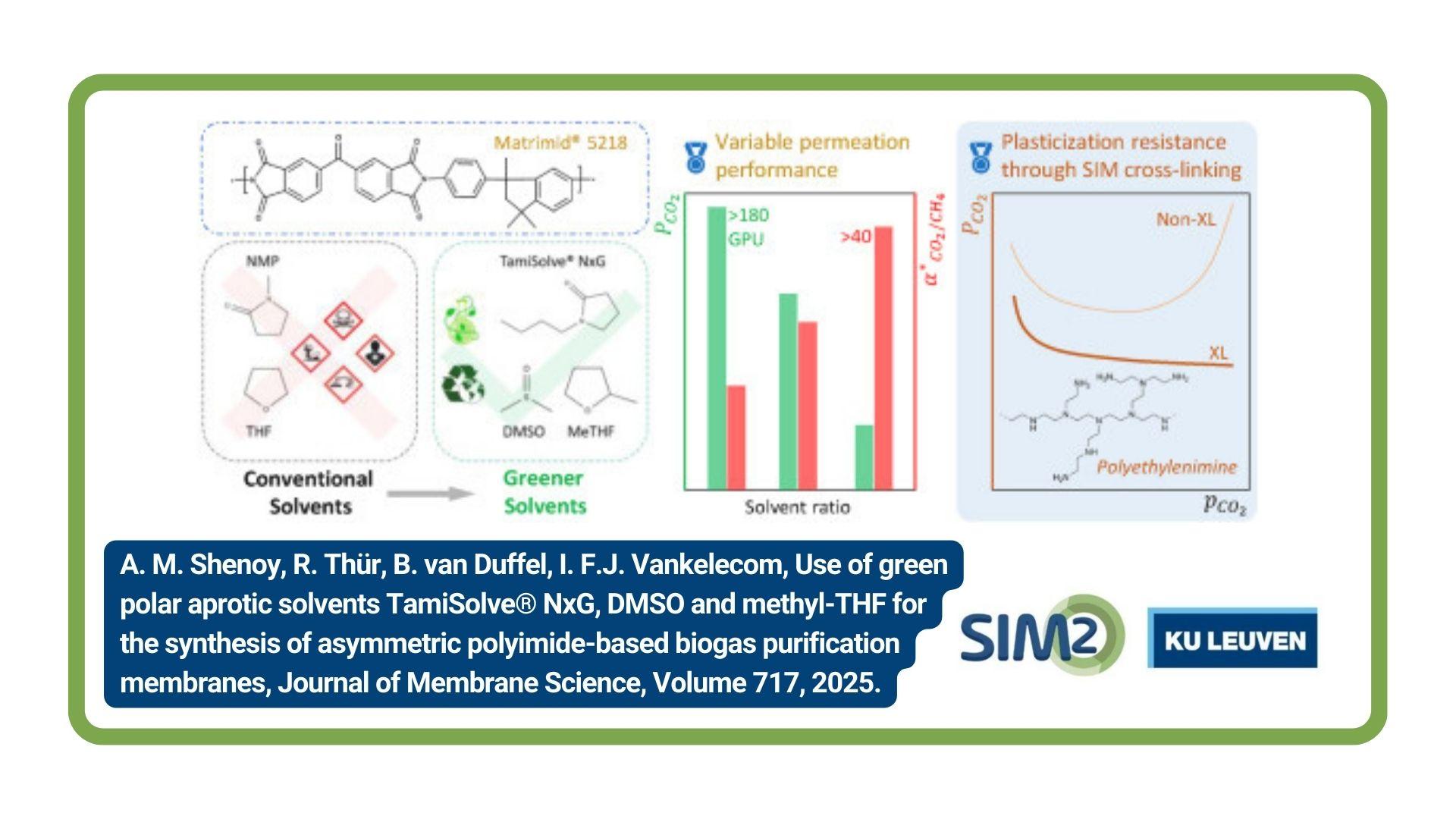
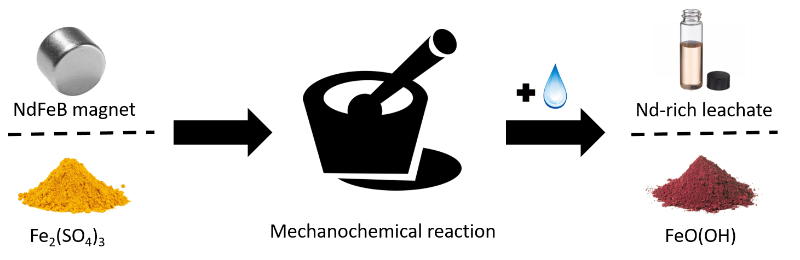
Researchers from the ProcESS and SOLVOMET Group have developed a mechanochemically intensified acid-free leaching process for the recovery of critical metals from NdFeB magnets, showing that cheap ferric sulfate can be employed for recovery of critical metals (rare-earth elements and Co). The work has been published in the journal Hydrometallurgy.
The transition to a low-carbon economy has promoted ‘clean-tech’ applications, such as wind turbines, computers, electric/hybrid cars and electric bikes. All these applications rely on NdFeB permanent magnets, which has made these magnets one of the largest and fastest growing appliances in the market among all rare-earth elements (REEs).
Traditionally, hydrometallurgical methods are used as they are generally applicable to all types of magnet compositions by dissolving in strong mineral acids, e.g. H2SO4, similar to REE processing from primary ores. The disadvantages of the large consumption of chemicals, in particular mineral acids, and the large volumes of secondary waste are minimized by combining hydrometallurgical leaching with a pyrometallurgical pre-treatment. However, these pre-treatments require high temperatures often resulting in high pressures, pollutants (e.g. SO2 or NOX) and high capital expenditures.
Intensified leaching of critical metals using mechanochemistry
Recently, mechanochemistry has been increasingly investigated as alternative to address waste recycling difficulties such as low solubility or high chemical consumption. A proof-of-principle was given for the recovery of rare-earth elements and Co from end-of-life NdFeB magnets in which ferric sulfate can be used as alternative to mineral acids, resulting in a high recovery (>95%) of rare-earth elements and full recovery of cobalt. A low-intensity mechanochemical milling operation, prior to leaching, not only improves the leaching yield and selectivity over iron but also reduces the ferric sulfate consumption. The whole process consumes only ferric sulfate, water and oxalic acid and produces a mixed REE-oxide precipitate, an iron precipitate (goethite and lepidocrocite) and an acidic leachate stream containing high concentrations of Fe(II). The process can be operated in closed loop by converting the remaining Fe(II) to Fe(III), towards a near-zero waste process.
Full reference paper Recovery of valuable metals from NdFeB magnets by mechanochemically assisted ferric sulfate leaching. Steff Van Loy, Mehmet Ali Recai Önal, Koen Binnemans and Tom Van Gerven. Hydrometallurgy (191), 105154. (Open Access).
Acknowledgements
This research is supported by an SB PhD fellowship (1S23518N) of FWO-Flanders. This work has received funding from the European Union's Horizon 2020 Research and Innovation Programme under grant agreement No 720838 (NEOHIRE).