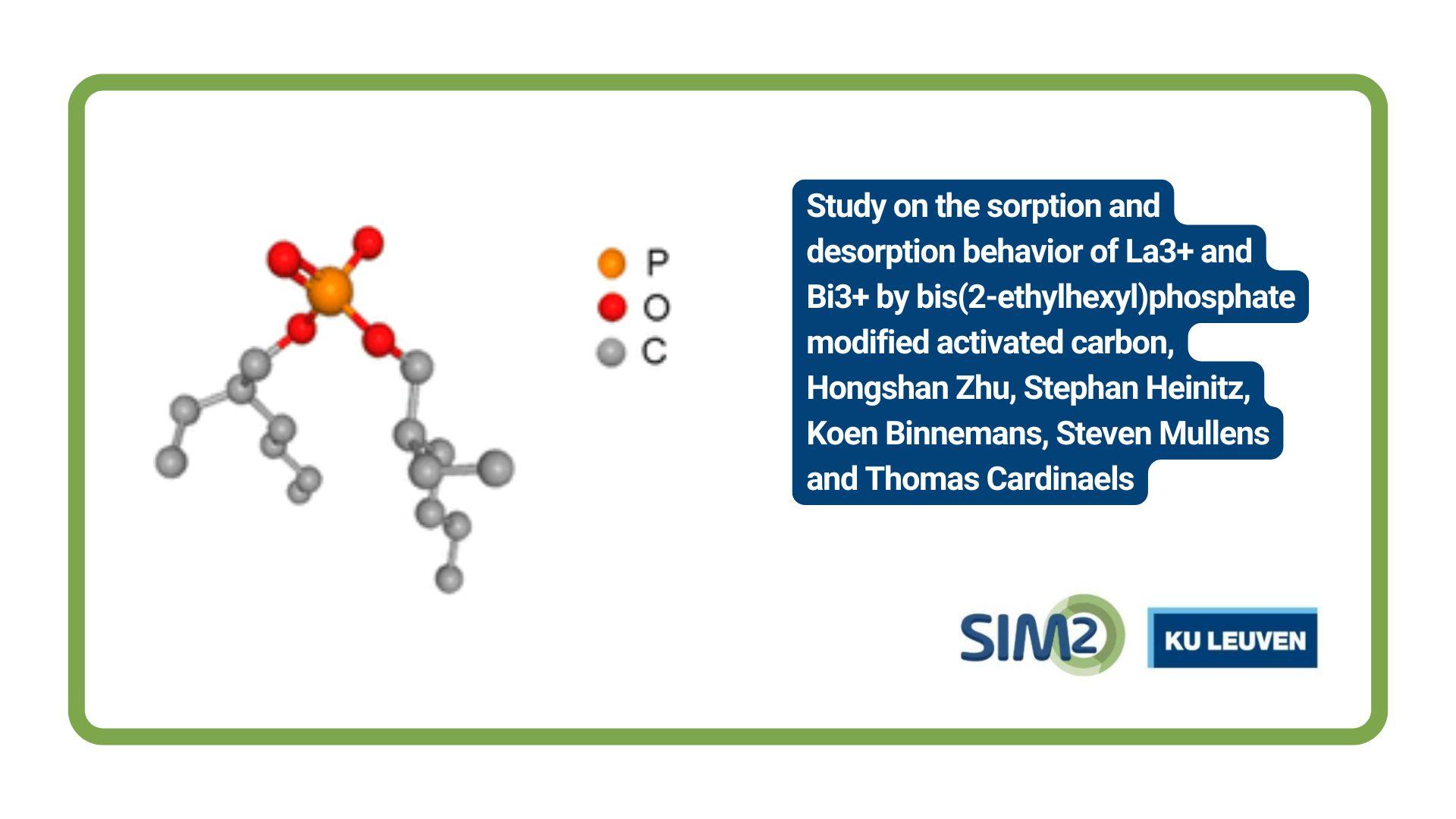
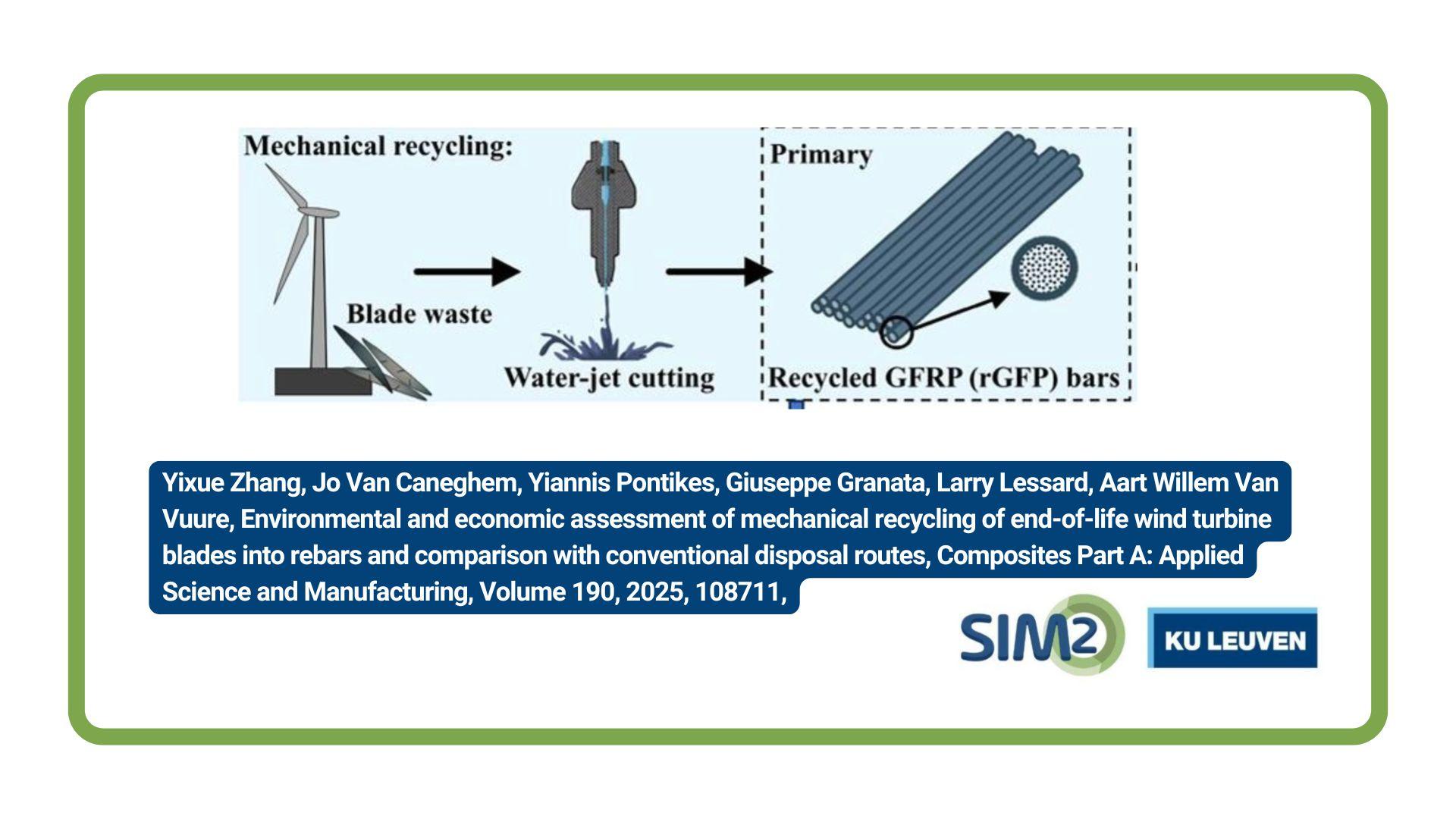
SIM² KU Leuven – SOLVOMET/SURF researchers unraveled the electrodeposition behaviour of gallium in a non-aqueous electrolyte, comprising of gallium(III) chloride and 1,2-dimethoxyethane (DME). The authors were given the opportunity to design the front cover of the journal Physical Chemistry Chemical Physics (PCCP, issue 29/2021).
Why gallium?
Gallium is metal that is indispensable for the electric and electronic equipment industry because of its use in a variety of semiconductors that serve as principal components in integrated circuits, mobile phones, light-emitting diodes, photovoltaics devices and detectors. These semiconductors require particularly high-purity gallium as even the smallest impurities might alter their properties and functionality. To refine gallium to such purity, various methods have been explored. Once such method is electrodeposition.
Electrodeposition of gallium
In aqueous solutions, the electrodeposition of gallium metal is inefficient, as the process competes with the electrochemical reduction of water (the hydrogen evolution reaction).
Consequently, electrodeposition of gallium from more electrochemically stable non-aqueous electrolytes has been investigated. Although efficient gallium electrodeposition has been achieved from multiple non-aqueous systems, the underlying electrochemical and deposition processes are typically not well understood.
Using non-aqueous electrolytes
In this study, we methodically investigated the electrochemical behaviour and the electrodeposition mechanism of gallium from an electrochemically stable non-aqueous electrolyte comprised of gallium(III) chloride and 1,2-dimethoxyethane.
Using combined techniques, we demonstrate that the reduction process occurs in two steps, in which gallium(I) is formed as an intermediate species. Electrodeposition of gallium is achieved, and it is shown that metallic gallium is electrodeposited as spheres with diameters of approximately two hundred nanometers that are stacked on top of each other.
X-ray photoelectron spectroscopy reveals that each gallium sphere is covered by a nanometer-thin gallium oxide shell. A series of electrochemical experiments indicate that these oxide shells are electrically conductive, and that a droplet-on-droplet deposition mechanism is followed.
Furthermore, a mechanism is postulated and subsequently experimentally proven for the formation and growth of the individual spheres.
Full reference of paper
W. Monnens, P.C. Lin, C. Deferm, K. Binnemans, J. Fransaer, Electrochemical behavior and electrodeposition of gallium in 1,2-dimethoxyethane-based electrolytes, Physical Chemistry Chemical Physics 23 (2021) 15492–15502. DOI: 10.1039/D1CP01074C
Acknowledgements
WM thanks the Research Foundation Flanders (FWO) for a PhD grant (1SB8319N). The research was supported by the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Programme: Grant Agreement 694078 – Solvometallurgy for critical metals (SOLCRIMET). The authors also want to thank Merve Kübra Aktan for her assistance with the SEM measurements.
.png)