SOLVOMET members have developed a closed-loop, green, and environmentally-friendly process to
recover REEs from lamp phosphor waste using methanesulfonic acid (MSA). This new process can sequentially recover, with outstanding selectivity, three different lamp phosphor fractions using only MSA as lixiviant. The work has been published in Industrial Engineering and Chemistry Research, and it’s a clear example of the advantages of solvometallurgy for specific cases.
The industrial residue
End-of-life fluorescent lamps are an interesting source of rare earth elements (REEs) and are already collected in most countries because of their mercury content. Currently, this residue is processed for the removal of the mercury, the magnetic fraction and the glass particles.
The remaining solid is the lamp phosphor waste powder, which is rich in REEs and is generally stockpiled. The lamp phosphor waste is composed of the HALO phosphor (45 wt%, does not contain REEs), the YOX phosphor (10-20 wt%, rich in yttrium and europium), and the LAP phosphor (6-7 wt%, rich in lanthanum, cerium and terbium), amongst other fractions.
The recovery process
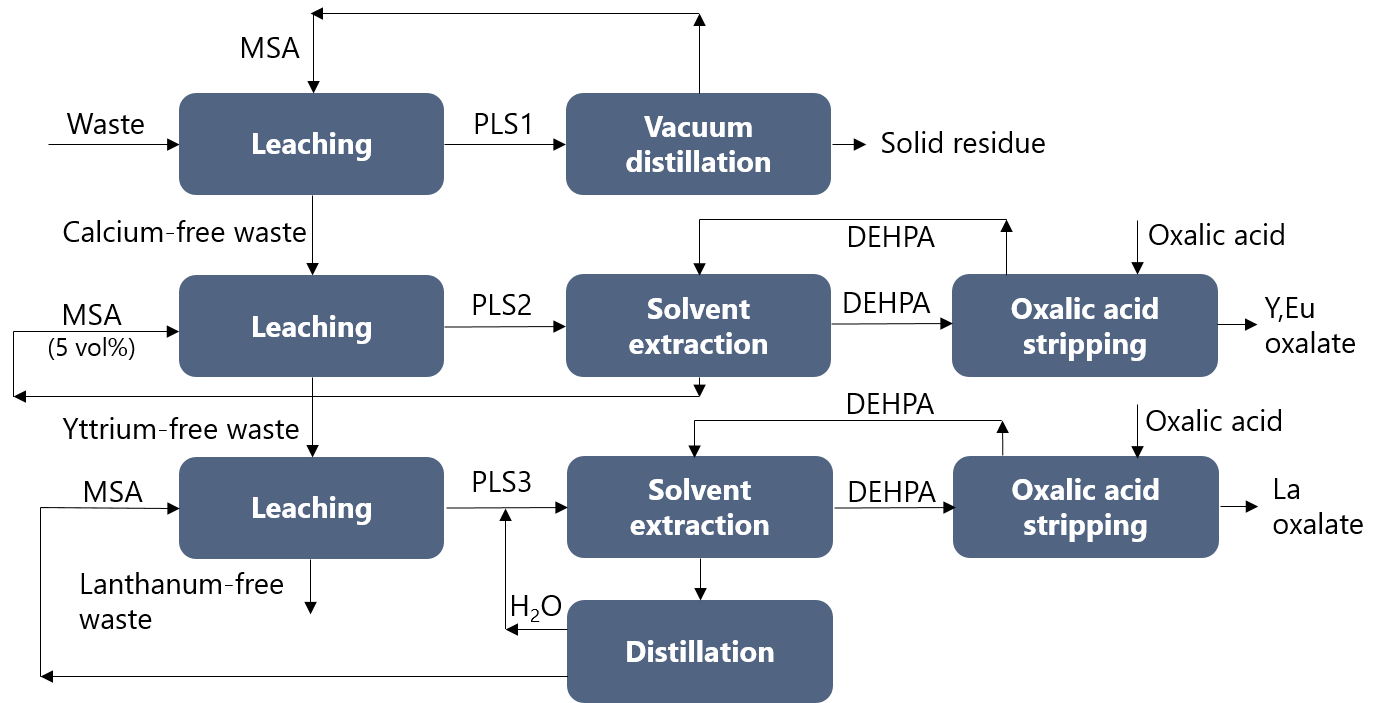
The REEs of the lamp phosphor waste can be recovered via hydrometallurgical methods. The first step is the dissolution of the HALO phospor, but the co-dissolution of the YOX phosphor is unavoidable. This is a problem because the HALO phosphor does not contain any REE, whereas the YOX phosphor has the highest intrinsic value.
Solvometallurgy can be an alternative for hydrometallurgical processes with low selectivity. Methanesulfonic acid (MSA) has previously proven to easily dissolve metal oxides and other mineral phases with outstanding selectivity. The solubility and the selectivity of the metals could be controlled by modifying the water content of the lixiviant.
The solubility of the HALO, YOX, and LAP phosphors was found to be largely affected by the MSA concentration and the leaching temperature. These two parameters were appropriately modified to selectively leach the HALO, the YOX and the LAP phosphors. Pure MSA could selectively leach all the HALO phosphor at low temperatures, with no co-dissolution of the YOX phosphor.
The calcium-rich PLS was purified by vacuum distillation, and both calcium and the formed phosphoric acid were recovered. The calcium-free residue was leached with diluted MSA to selectively leach the YOX phosphor. The remaining residue could be leached with pure MSA at elevated temperatures to recover the LAP phosphor. The yttrium-rich PLS and the lanthanum-rich PLS were purified via solvent extraction with DEHPA and stripped via oxalic acid precipitation to produce a highly pure yttrium/europium or lanthanum oxide product. The devloped process is summarised in the following Figure.
Full reference of paper
N. Rodriguez Rodriguez, B. Grymonprez, K. Binnemans, Integrated process for recovery of rare-earth elements from lamp phosphor waste using methanesulfonic acid, Industrial & Engineering Chemistry Research 60 (2021) 10319–10326, DOI: 10.1021/acs.iecr.1c01429
Acknowledgements
N.R.R. acknowledges the financial support from the Research Foundation-Flanders (FWO, grant no. 12X5119N, postdoctoral postdoctoral fellowship). The authors acknowledge Relight Srl (Rho, Italy) for providing the lamp phosphor waste.
.png)