Ganesh Pilla and colleagues from the KU Leuven, Department of Materials Engineering have published a new article on the reactions and phase transformations during the lo-temperature reduction of bauxite residue by hydrogen in the presence of NaOH, and recovery rates downstream. The article was published in the Proceedings of the 40th International ICSOBA Conference, Athens, 10-14 October 2022.
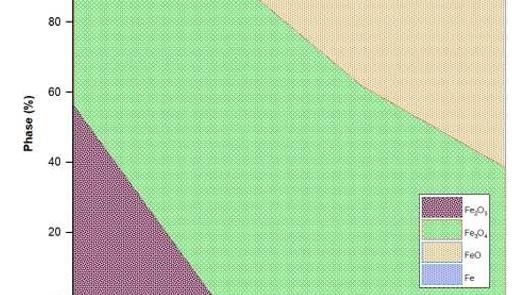
The scope of this work is to investigate the reactions and phase transformations that occur during the low-temperature reduction of bauxite residue (BR) by hydrogen in the presence of NaOH, and how that affects the recovery downstream of the different elements. Different processing conditions were studied, such as temperature, time, and NaOH dosage under a pure hydrogen atmosphere. XRD results revealed that hematite (Fe2O3) and goethite (FeOOH) were completely converted to magnetite (Fe3O4) when a blend of BR and 20 wt % NaOH was treated at 500 °C for 30 min. The iron recovery and grade of magnetic rich fraction after water leaching -magnetic separation of reduced BR (at 500 °C, 20 wt % NaOH, 30 min reduction time) is 87.7 % and 44.5 % respectively, whereas the aluminum and sodium recovery under these conditions is 75 % and 87 %. The formation of NaAlO2 occurs at 400 °C, and the amount formed increases till 700 °C. When NaOH addition exceeds 25 wt % and the temperature is higher than 600 °C, the formation of water-soluble sodium iron oxide (NaFeO2) and sodium-calcium silicate (Ca2Na2Si3O9) along with wüstite (FeO) takes place. This led to lower Fe and Al recovery and further dissolution of Fe, Si, and Ca in the leach liquor during water leaching and wet-magnetic separation. For thermal processing at 500°C for 30 min with 20 wt % NaOH, the amount of Fe3O4 forming is the highest, and via magnetic separation, a Fe-rich fraction is recovered. In the non-magnetic fraction, perovskite (CaTiO3), calcite (CaCO3), and quartz (SiO2) are enriched, whereas the leach liquor is Na and Al-rich, as confirmed by ICP-OES measurements. The partitioning of rare earth elements in the different phases, as well as what is overall the preferable recovery process, are also discussed.
Reference
Reactions and Phase Transformations During the Low-Temperature Reduction of Bauxite Residue by H2 in the Presence of NaOH, and Recovery Rates, G Pilla, T Hertel, S Kapelari, B Blanpain, Y Pontikes link to article
Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 958307.