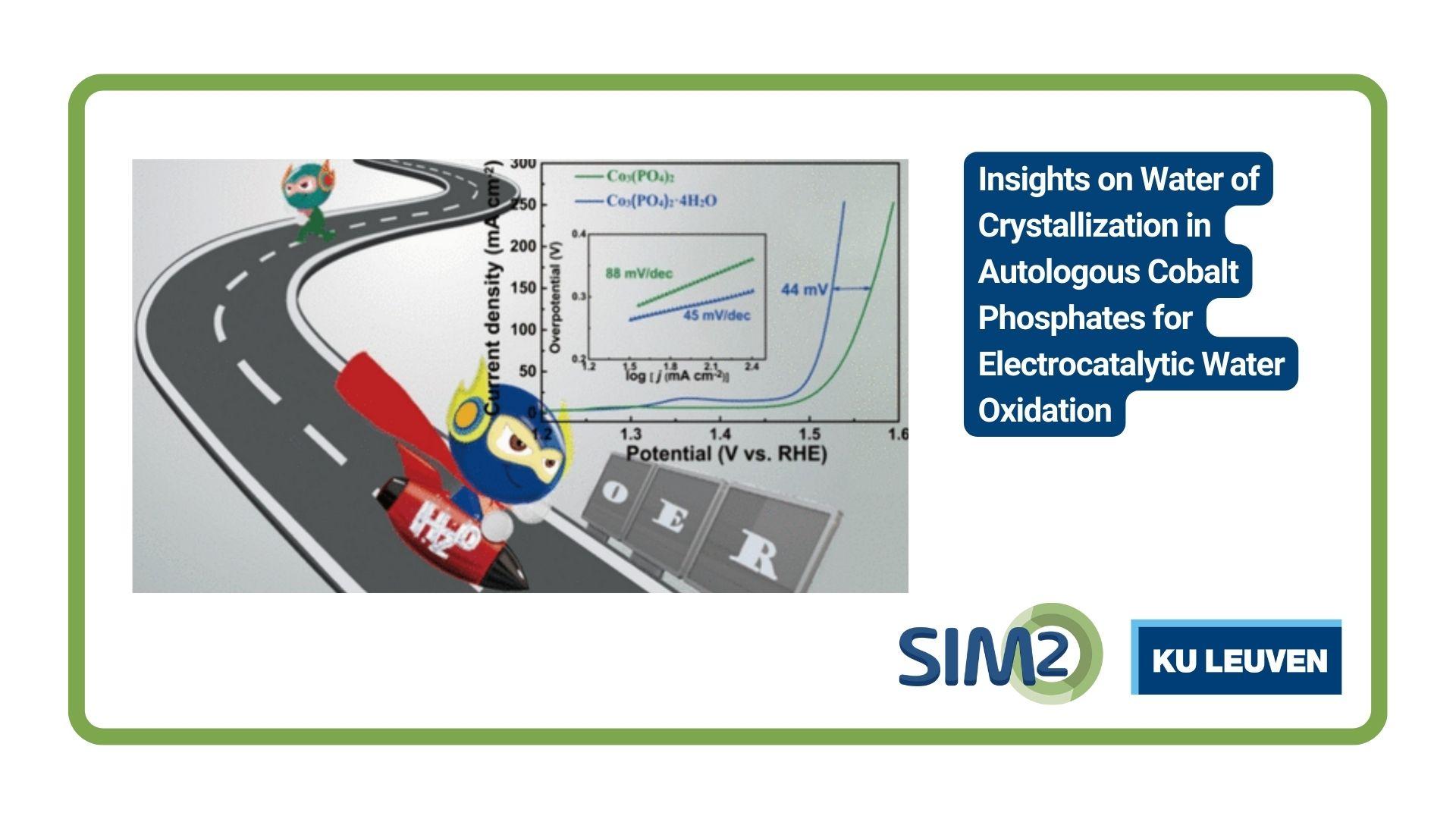
Jan Fransaer of the Department of Materials Engineering, KU Leuven, together with colleagures from China, Australia and Belgium, has published a new artcie on the water of crystallization in autologous cobalt phosphates for electrocatalytic water oxidation. The article is published in the June 26th edition of ACS Materials Letters.
Cobalt phosphates are efficient electrocatalysts for the oxygen evolution reaction (OER), the rate-determining step in water splitting. However, the role of water crystallization in OER electrocatalysis is not well understood, limiting the systematic design of such catalysts. This study compares Co3(PO4)2·4H2O (CoPO-25) and Co3(PO4)2 (CoPO-25NW) nanoflakes to highlight the importance of water of crystallization. The results show that water of crystallization increases the oxygen vacancy concentration, enhances electrical conductivity, and modifies electronic structure, leading to higher catalytic activity for CoPO-25. Additionally, the water of crystallization sustains the structural stability of CoPO-25, preventing complete hydroxylation conversion. Density functional theory calculations reveal that the water of crystallization in CoPO-25 is crucial for modifying binding interactions between reaction intermediates and the catalyst. This study not only presents a promising electrocatalyst but also provides insights into the impact of water crystallization in cobalt phosphates on the OER.
Reference
Insights on Water of Crystallization in Autologous Cobalt Phosphates for Electrocatalytic Water Oxidation, Wei Zhang, Renji Zheng, Ning Han, Wei Guo, Qiong Liu, Zhijie Chen, Yuhai Dou, Xuan Zhang, Jiangshui Luo, and Jan Fransaer, ACS Materials Letters Article ASAP, DOI: 10.1021/acsmaterialslett.4c00853