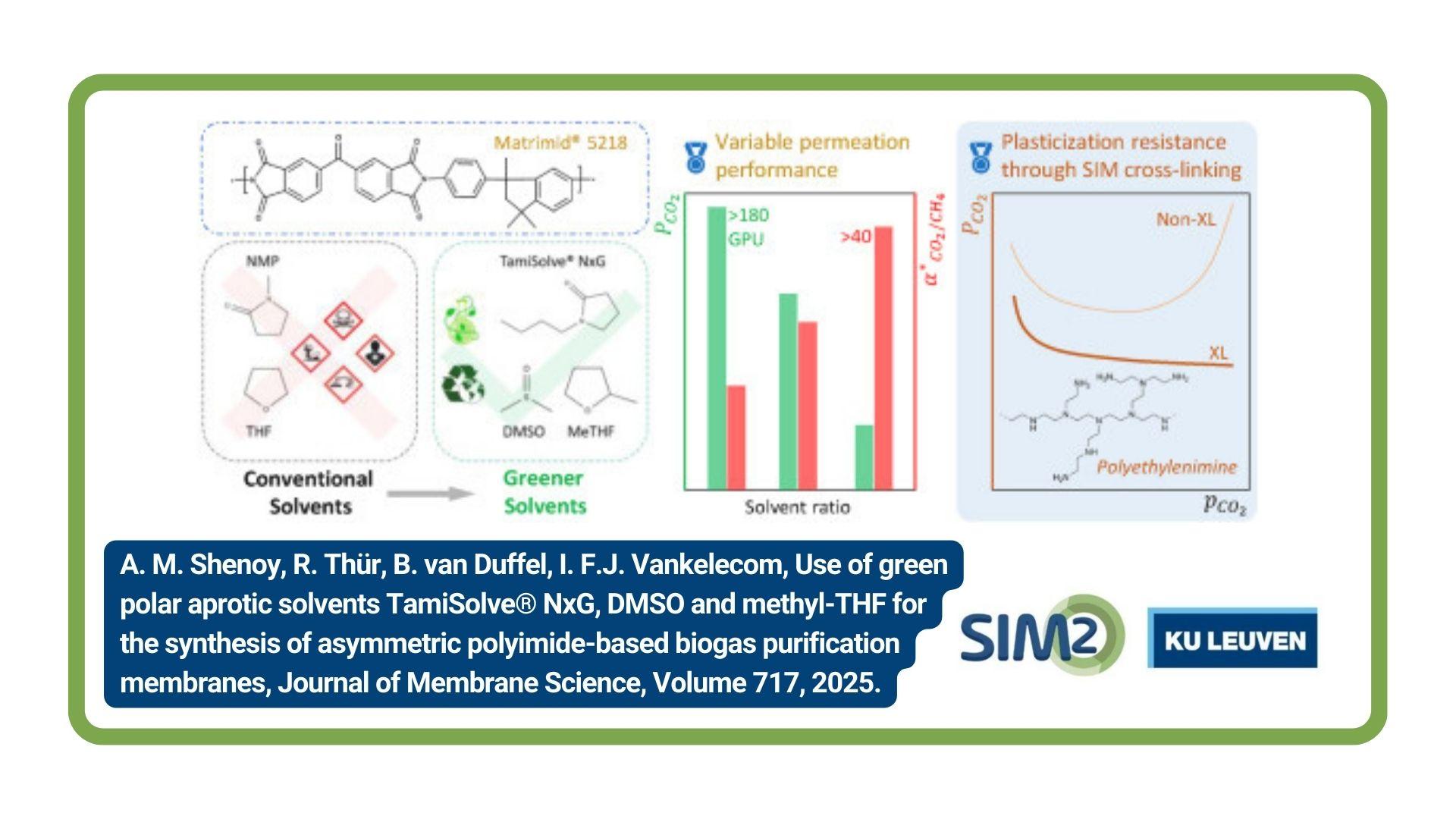
Within the context of the EU H2020 TARANTULA project, SOLVOMET/SIM² KU Leuven researchers developed a conceptual flowsheet for the non-aqueous recovery of tungsten from low-grade scheelite sources without formation of tungsten solid intermediates. Other advantages are: selective solvent extraction of tungsten; mild leaching condition at atmospheric pressure and temperature <100 °C; no generation of high-salinity waste water. The work was published in the journal Industrial and Chemical Engineering Research.
Tungsten has been consistently included in the various lists of critical raw materials as published by the European Commission. More specifically, tungsten is the metal with the highest economic importance for its unique performance in strategic industrial large-base applications (metal-work tools) as well as in emerging technologies (robotics, drones, 3D-printing).
Tungsten extraction
Scheelite (CaWO4) and wolframite ((Fe,Mn)WO4) are the two main tungsten ores, with scheelite being the easiest to dissolve. The state-of-the-art process to extract tungsten from scheelite ores has changed very little throughout the years. The core step in the flowsheet of the conventional industrial processing of scheelite is digestion with NaOH or Na2CO3 to convert CaWO4 into Na2WO4, followed by the removal of impurities and acidification of the Na2WO4 solution (pH 2–3).
The disadvantages of this industrial process are the formation of solid intermediates which necessitate an additional dissolution step, harsh process conditions (process temperature and pressure conditions above 100 °C and 1 atm, respectively), almost zero recycling of the chemicals and large production of high-salinity wastewater (25 tonnes per tonne of produced tungsten product).
New solvometallurgical process for tungsten recovery
The idea of the SOLVOMET/SIM2 KU Leuven team was to shift the CaWO4 dissolution to a non-aqueous medium, which is able to solvate the formed H2WO4. Solutions of 2 mol L-1 aqueous HCl in ethylene glycol (EG), polyethylene glycol-200 (PEG-200), acetonitrile, Aliquat 336 (all with a water content <12 vol.%) were prepared and compared with an equivalent HCl aqueous solution for the dissolution of high-grade (55 wt%) and low-grade (3.3 wt%) CaWO4.
Full dissolution of CaWO4 without precipitation of the formed H2WO4 was possible in the EG and PEG-200 solutions. EG was preferred as solvent given its lower viscosity in the following solvent extraction step at room temperature.
The stability of the solvents was investigated and the possible formation of 2-chloroethanol was discussed as well by the authors. The solvent extraction step was optimised investigating several solvents, extractants and salting agents as well as more polar on less polar phase ratios.
The best conditions for a 3-stage process for the selective extraction of tungsten comprise the use of Aliquat 336 in GS190 with 10 vol% 1-decanol and 2 mol L-1 CaCl2 as salting agent, followed by scrubbing of iron in water and stripping of tungsten in a 1 mol L-1 NH3/NH4Cl solution as ammonium paratungstate (APT, the most spread commercial tungsten product). Insights on the extractions mechanism of tungsten from a non-aqueous chloride medium were provided as well. The team plans to soon test the process in a mini-pilot plant, with a technology readiness level of TRL4-5. Future updates will be published on the H2020-RIA TARANTULA website.
Full reference of paper
Orefice, M., Nguyen, V.T. Raiguel, S., Jones, P.T., Binnemans, K. Solvometallurgical Process for the Recovery of Tungsten from Scheelite. Industrial and Chemical Engineering Research. (2021). https://doi.org/10.1021/acs.iecr.1c03872
Acknowledgements
The research leading to these results has received funding from the European Commission’s Horizon 2020 Programme ([H2020/2014-2019]) under Grant Agreement no. 821159 (H2020–RIA TARANTULA). This publication reflects only the authors’ view, exempting the Community from any liability.