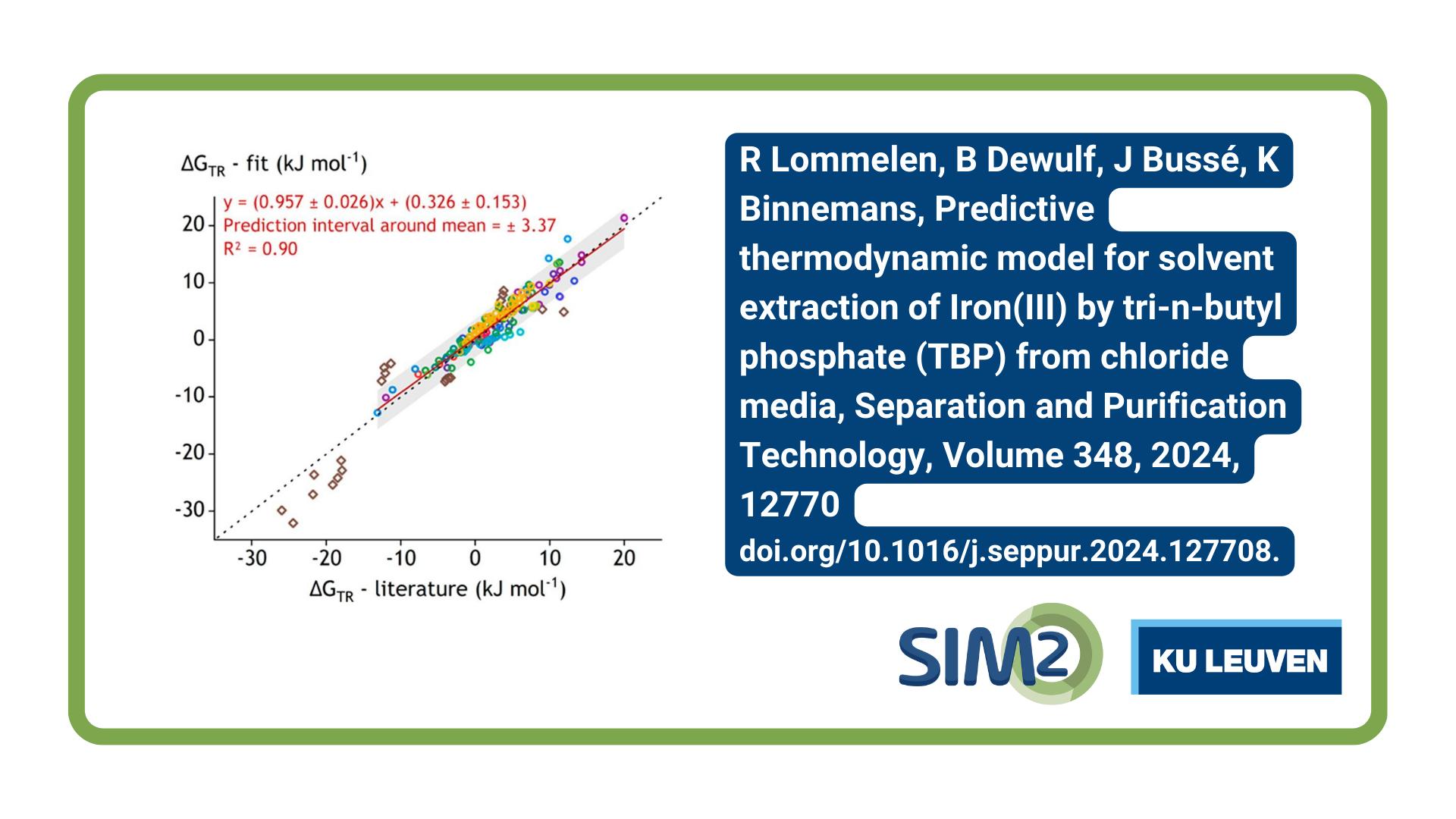
Rayco Lommelen, Brecht Dewulf, Jakob Bussé, and Koen Binnemans from the SOLVOMET Group at KU Leuven have published a paper in the journal Separation and Purification Technology that introduces a cutting-edge semi-empirical predictive thermodynamic model for iron removal from chloride media using solvent extraction with tri-n-butyl phosphate (TBP).
Hydrometallurgical processes are crucial to reducing the environmental impact of metal recovery, yet dealing with iron impurities is a major challenge. This paper presents a semi-empirical predictive thermodynamic model for iron removal from chloride media by solvent extraction with tri-n-butyl phosphate (TBP). This model is built upon the OLI mixed-solvent electrolyte framework (OLI-MSE). It goes beyond traditional thermodynamic models by incorporating the non-ideality of both the aqueous and organic phases in a single, chemistry-based thermodynamic model. On top of the OLI-MSE framework lies the chemical model that depicts the extraction of Fe(III) by forming an ion pair between the FeCl4– anion and protonated TBP (TBPH+) in the organic phase. The iron(III)-containing ion pair in the organic phase is further stabilized by interactions with neutral TBP molecules. Developing the model required modeling the Fe(III) – chloride chemistry in the aqueous phase, optimizing the HCl extraction model, and fitting the parameters between FeCl4–, TBPH+, and TBP to experimental solvent extraction data. The resulting thermodynamic model can predict the solvent extraction of iron(III) from complex feed solutions by TBP in aliphatic diluents at all concentrations up to undiluted TBP. It can be used to calculate the equilibrium energies and composition, and the species present at equilibrium.
Reference
Rayco Lommelen, Brecht Dewulf, Jakob Bussé, Koen Binnemans, Predictive thermodynamic model for solvent extraction of Iron(III) by tri-n-butyl phosphate (TBP) from chloride media, Separation and Purification Technology, Volume 348,
2024, 127708, ISSN 1383-5866, https://doi.org/10.1016/j.seppur.2024.127708.
Acknowlegements
This work was supported by the European Union’s Framework Programme for Research and Innovation Horizon Europe [Grant number 101058124 (ENICON)]. We thank Dr. Tim Balcaen (KU Leuven) for his valuable help and insights when performing the statistical analysis on the agreement between the thermodynamic model and the experimental data.