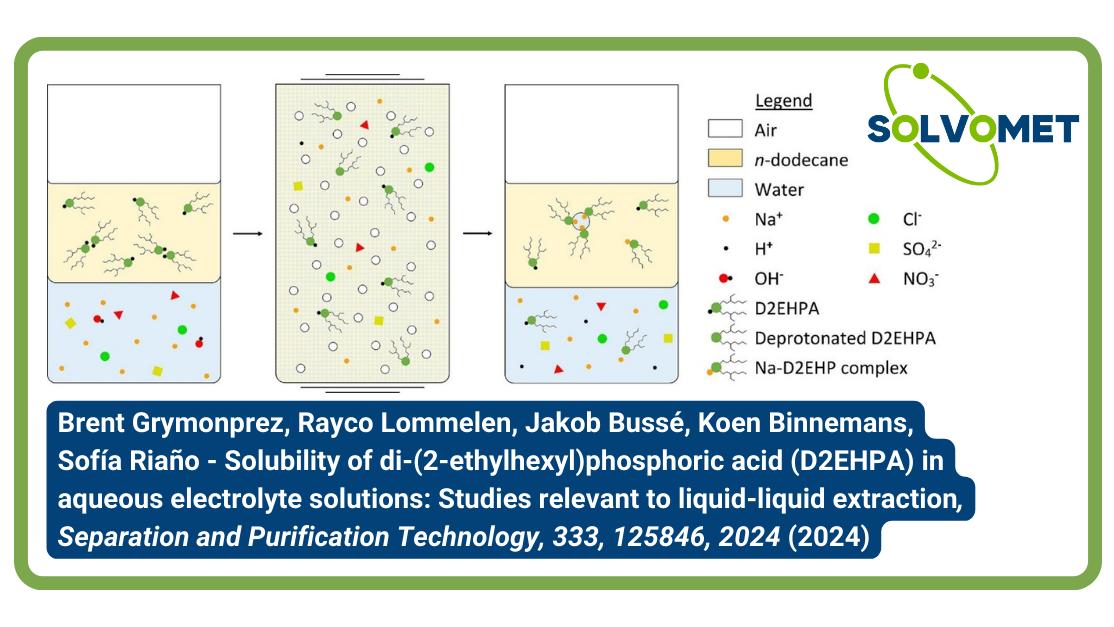
Di-(2-ethylhexyl)phosphoric acid (D2EHPA) is a commonly used acidic extractant in solvent extraction for metal refining. Limited information exists regarding the impurities in commercially available D2EHPA and their influence on liquid-liquid extraction. This study identifies these impurities, emphasizes the importance of preconditioning the extractant before extractions, and investigates D2EHPA solubility in aqueous solutions as a function of pH, extractant concentration, and anion presence (chloride, bromide, iodide, sulfate, methanesulfonate, thiocyanate, and nitrate). The study also examines the density of organic phases and sodium and water uptake in the organic phase concerning different aqueous phase anions. Anions were found to have minimal impact on D2EHPA solubility. Low salt concentrations or high pH values led to significant D2EHPA losses to the aqueous phase. A notable increase in water content in the organic phase occurred around pH 5 (pH 6 for the nitrate system), accompanied by increased sodium ion extraction, explaining higher organic phase density and a tendency toward third-phase formation. These findings aid in understanding D2EHPA behavior in liquid-liquid extraction systems, preventing third-phase formation and extractant losses to the aqueous phase. Additionally, the study evaluates impurities in commercially available D2EHPA and their impact on liquid-liquid extraction processes.
Key Takeaways:
- The D2EHPA solubility is significantly influenced by the pH and salt concentration.
- The solubility of D2EHPA in the aqueous phase is impacted by the concentration of D2EHPA in the organic phase.
- The sodium uptake and organic phase density are strongly influenced by pH.
To establish an economically viable solvent extraction process, it is essential for the extractant to have low solubility in the aqueous phase and understanding the factors influencing this solubility is equally crucial. Di-(2-ethylhexyl)phosphoric acid (D2EHPA) is a widely used acidic extractant due to its selectivity, versatility, commercial availability, favorable physicochemical properties, and chemical stability. Over the years, D2EHPA has played a pivotal role in separating rare-earth elements, nickel and cobalt, indium and gallium, zinc, vanadium, and iron.
In the context of a liquid–liquid extraction process, the solubility of D2EHPA depends on three primary factors. Firstly, the concentration of salts in the aqueous phase plays a key role, as the salting-out effect aids in retaining the organic extractant in the organic phase. Secondly, the pH of the aqueous solution is significant, as elevated pH values lead to the deprotonation of the extractant, causing the extractant anion and its counter cation to migrate to the aqueous solution. Lastly, temperature influences solubility, with the general trend being an increase in the solubility of organic components in the aqueous phase as temperatures rise.
Despite the importance of these factors, there is a scarcity of studies focusing on the solubility of D2EHPA in aqueous mixtures, and some available data are at times contradictory. In this study, we examine the sodium-D2EHPA-n-dodecane system, which has been insufficiently documented despite its constant use in solvent extraction research. Our investigation focuses on the solubility of D2EHPA in the aqueous phase, as well as the density, sodium content, and water content of the organic phase. These parameters are studied as a function of pH, the anion of the sodium salt, salt concentration, temperature, and D2EHPA concentration. To enhance the precision of extractant loss estimations attributable to solubility and gain a deeper understanding of the factors influencing third-phase formation in liquid–liquid extraction, we also identify and quantify impurities present in commercially available D2EHPA. The impact of these impurities on liquid–liquid extraction processes is thoroughly examined and discussed. This comprehensive analysis provides valuable insights that contribute to a more accurate assessment of extractant losses and a better comprehension of the dynamics influencing third-phase formation in liquid–liquid extraction processes.
Full reference
Brent Grymonprez, Rayco Lommelen, Jakob Bussé, Koen Binnemans, Sofía Riaño, Solubility of di-(2-ethylhexyl)phosphoric acid (D2EHPA) in aqueous electrolyte solutions: Studies relevant to liquid-liquid extraction, Separation and Purification Technology, 333, 125846, 2024, https://doi.org/10.1016/j.seppur.2023.125846
Acknowledgements
This research was possible thanks to the European Research Council (ERC) – European Union’s Horizon 2020 Research and Innovation Programme: Grant Agreement 694078—Solvometallurgy for Critical Metals (SOLCRIMET).