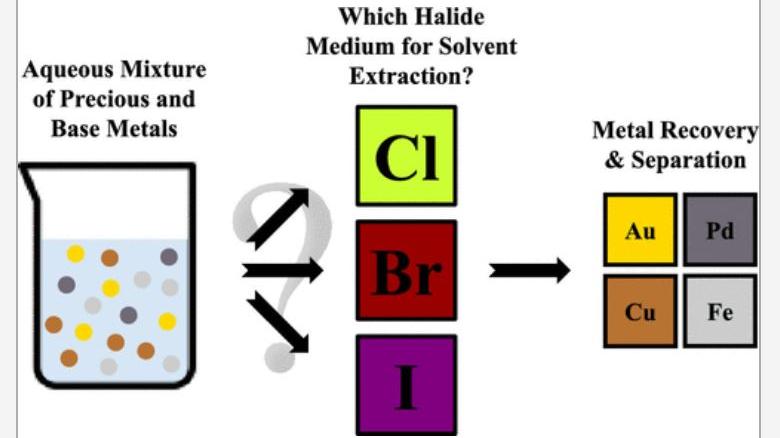
SIM² KU Leuven – SOLVOMET researchers studied how a variation of the halide extraction medium affects the separation of precious and base metals using the undiluted ionic liquid Aliquat 336. Based on the obtained results, a separation process was developed for the purification of small amounts of gold and palladium from copper and iron rich solutions. The developed process can be considered relevant for the recycling of waste electric and electronic equipment (WEEE). The work has been published in the journal ACS Sustainable Chemistry & Engineering.
Unconventional solvent extraction (SX) systems
In recent years, a lot of research efforts have been directed to the reduction of the environmental impact of metallurgical processes. Among the newly developed technologies is the implementation of undiluted ionic liquids for the solvent extraction of metal ions.
Ionic liquids, being non-volatile and non-flammable, are considered safer and more environmentally friendly than conventional organic diluents and have shown excellent selectivity and efficiency in various metal separation applications.
Another interesting research line is the development of bromine and iodine based leaching methods for precious metals, which show high leaching rates and an increased selectivity for the separation of precious and base metals.
However, solvent extraction research has focused primarily on the use of conventional (i.e. chloride, nitrate and sulfate media) extraction media and only little is known about the alternative halides. This prompted the question which was the starting point of this research: How would such bromide or iodide media influence the extraction behavior and separation possibilities of dissolved metal ions using undiluted halide ionic liquids?
Results
The extraction behavior and separation possibilities of several precious and base metal ions, i.e. Pt(IV), Pd(II), Rh(III), Au(III), Cu(II), Fe(III), and Ni(II), from the different halide media was explored using the undiluted ionic liquid Aliquat 336 chloride and its bromide and iodide analogues.
Apart from a reversed extraction order for Fe(III) and Cu(II), the chloride and bromide systems behaved similarly and showed promising characteristics for the separation of Pt(IV), Pd(II), and Au(III) from Cu(II), Fe(III), and Ni(II). The iodide system allowed for a very efficient, single-step separation between the precious metal ions Pd(II) or Pt(IV) and the base metal ions Fe(II) or Ni(II).
The comparison of the chloride and bromide systems was continued through the development of a separation flowsheet. Synthetic solutions were prepared containing very little Au(III) and Pd(II) but large amounts of Cu(II) and Fe(III).
The composition of these solutions was based on the composition of waste electric and electronic equipment (WEEE), one of the largest and fastest growing waste streams worldwide. Proper handling and recycling of this rapidly growing waste stream is imperative for both the environment and human health but can also be interesting from an economical point of view, as it can be an important source of both base and precious metals.
WEEE can contain a whole range of metals, but iron and copper are its main constituents by weight. The precious metals gold and palladium are present in only minor amounts, even in high grade scrap, but they represent 70-80% of the value share.
In terms of extraction performance and scrubbing characteristics, the chloride and bromide media performed similarly allowing an easy separation of Au(III) and Pd(II) from Cu(II) and Fe(III) through an extraction at low halide concentrations and scrubbing with water.
However, the bromide system showed a superior stripping behavior, providing an easy and quantitative separation and recovery of Au(III) and Pd(II) using ammonia and sodium sulfite respectively.
Future use in SX research
The performed research aims to contribute to the knowledge of alternative solvent extraction media. The fact that the bromide system allowed for a more efficient metal separation in this case study underlines the usefulness of broadening solvent extraction research to media other than the conventional systems.
Full reference of paper:
Vereycken, W.; Riaño, S.; Gerven, T. V.; Binnemans, K. Extraction Behavior and Separation of Precious and Base Metals from Chloride, Bromide, and Iodide Media Using Undiluted Halide Ionic Liquids. ACS Sustainable Chem. Eng. 2020, 8 (22), 8223–8234. https://doi.org/10.1021/acssuschemeng.0c01181.
Acknowledgements
The authors thank the KU Leuven for financial support (Project C24/18/042, ISOMER).