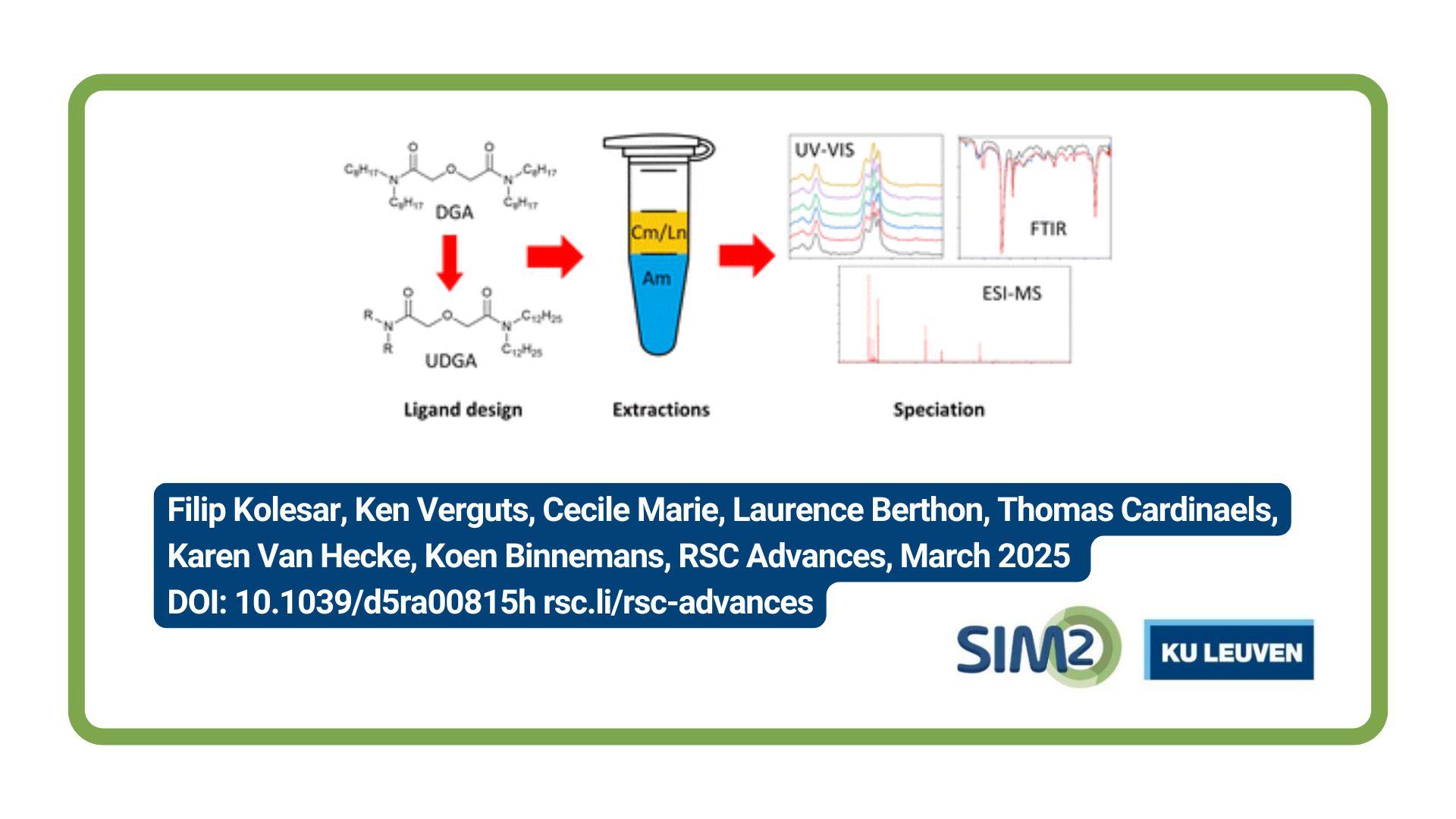
Filip Kolesar and colleagues from the KU Leuven, Department of Chemistry, the Belgian Nuclear Research Center, and the University of Montpellier have published a new article entitled Extraction and speciation studies of new diglycolamides for selective recovery of americium by solvent extraction. The article is published in the March issue of RSC Advances.
Much research has gone into the development of extraction processes capable of separating minor actinides from highly active raffinates generated by the PUREX process. In particular, the separation of americium from curium remains challenging because of the similarity of their chemical properties. A new class of diglycolamide extractants called “unsymmetrical” diglycolamides (UDGAs), which contain at least two different alkyl chains, have recently been shown to have potential for improving the Am/Cm selectivity. However, the influence of the alkyl chains on the extraction efficiency and selectivity, and the formed complexes are not fully understood yet. For this purpose, using the AmSel system as reference, six UDGAs were studied by performing both extraction experiments and speciation studies, and compared to the benchmark extractant TODGA. The tested UDGAs all contain two dodecyl chains on one of the amide nitrogen atoms, and either two n-propyl, isopropyl, n-butyl, isobutyl, or n-pentyl chains, or a piperidine group on the opposing nitrogen atom. The results show that all of the tested UDGAs have equal or higher distribution ratios than TODGA, with the isopropyl derivative showing the most efficient extraction of Ln(III) and An(III). Selectivity for curium over americium was equal or higher in comparison with TODGA, with isopropyl and piperidine giving the highest separation factors, followed by pentyl, and the remaining UDGAs showing similar selectivity to TODGA. Speciation experiments of the complexes formed in the organic phase with neodymium were performed using FTIR, ESI-MS, and UV-vis spectrometry. This revealed very similar spectra for all of the diglycolamides, indicating no difference in the extraction mechanism.
Reference
Filip Kolesar, Ken Verguts, Cecile Marie, Laurence Berthon, Thomas Cardinaels, Karen Van Hecke, Koen Binnemans, RSC Advances, March 2025 DOI: 10.1039/d5ra00815h rsc.li/rsc-advances
Acknowledgements
The authors would like to thank Dr Tomas Opsomer for his advice and assistance regarding the synthesis of the DGA compounds. The authors would also like to thank the LCIS and LILA laboratories at CEA for their assistance during experiments. The authors acknowledge the SCK CEN Academy for providing funding for a PhD fellowship and the FPS Economy for the support via the EnergyTransitionFund.Theauthorsalso acknowledge ENEN for their nancial assistance through the ENEN2plus mobility grant.