Researchers from the ChEMaRTS Research Group (Faculty of Engineering Technology at KU Leuven Campus Group T) have developed a method for the extraction of phosphorus from wastewater treatment sludge ash. The work has been published in the journal Science of the Total Environment.
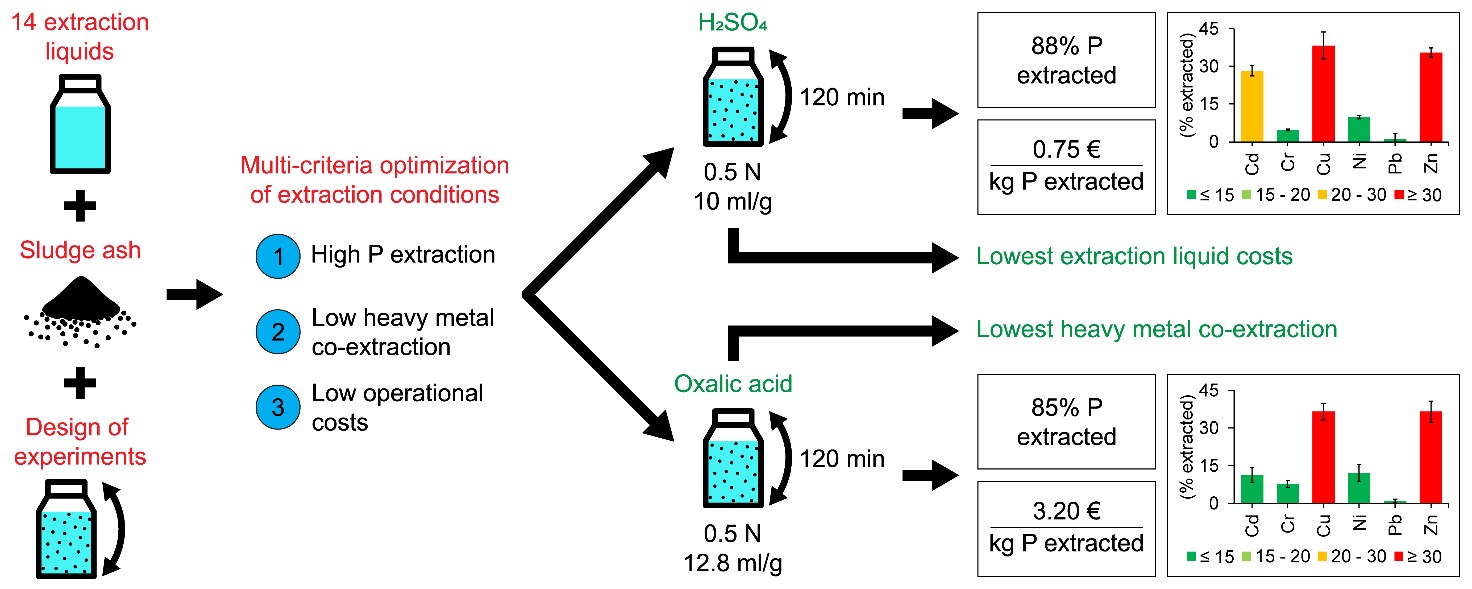
Direct application of wastewater treatment (WWT) sludge ash as a fertilizer is hindered by the presence of heavy metals in concentrations above legal limit values. Therefore, another option to recover the valuable phosphorus (P) in the sludge ash, and this way closing the P cycle, is a wet chemical extraction. Hence, the overall aim of this study was to establish optimal and cost-effective conditions for wet P extraction from WWT sludge ash by means of multi-criteria optimisation.
Optimal and cost-effective conditions refer to the best possible trade-off between high P extraction, low heavy metal (Cd, Cr, Cu, Ni, Pb and Zn) co-extraction and low operational costs. The use of design of experiments (DOE) allowed to evaluate the effect of multiple variables on the P and heavy metal extraction efficiency, i.e. extraction liquid concentration, liquid to solid (L/S) ratio and contact time for each of the fourteen extraction liquids considered.
Micronutrient
P is an essential micronutrient for all living organisms. This P is mostly extracted from phosphate rock, which is a non-renewable resource that is becoming scarce. Therefore, to meet future P demands, it is important to recover P from alternative sources, such as e.g. WWT sludge ash.
Chemical characterisation indicated that the Cd and Zn concentration in the sludge ash exceeded the Flemish legal concentration limit values for direct application of the ash as a fertiliser. Furthermore, XRD and SEM-EDX analysis showed that P in the sludge ash was mainly occurring as whitlockite and Al-phosphates. Based on the P extraction efficiency with a NaOH solution, it was estimated that around 40% of total P was bound as Al-phosphates, whereas the other 60% was probably bound as Ca-phosphates.
Results
DOE results indicated that P extraction efficiencies above the desired minimum of 85% were only obtained with H2SO4, HCl, HNO3 and oxalic acid solutions, whereas all other extraction liquids resulted in P extraction efficiencies below 50%. The type of extraction liquid, extraction liquid concentration, L/S ratio, contact time and interactions of the latter three variables all affected the P and heavy metal extraction efficiency. Results also showed that a P extraction efficiency > 85% can only be obtained at a pH < 2 at which both Ca- and Al-phosphates dissolve very well.
Additionally, results indicated that a high P extraction efficiency from the sludge ash coincides inevitably with high heavy metal co-extraction efficiencies. NaOH was the only extraction liquid dissolving almost no heavy metals, mainly because of the high pH at the end of the extraction procedure (12.5 – 13.0). However, the P extraction was low with NaOH (about 40%) due to the fact that Ca-phosphates are not or only slightly soluble in alkaline environment.
Multi-criteria optimisation
A multi-criteria optimisation indicated that from a techno-economic point of view, H2SO4 (0.5 N, 10 ml/g, 120 min) and oxalic acid (0.5 N, 12.8 ml/g, 120 min) showed the best trade-off between high P extraction, low heavy metal (Cd, Cr, Cu, Ni, Pb and Zn) co-extraction and low operational costs. H2SO4 had the lowest extraction liquid cost per kg P extracted, whereas oxalic acid had the lowest heavy metal co-extraction, reducing the number and costs of downstream processing steps. It should, however, be noted that for a final selection of extraction liquid, the environmental impact, overall sustainability and total costs of the P recovery process have to be considered in an extended Life Cycle Analysis and Techno-Economic Assessment.
Full reference of the paper
Luyckx, L., Geerts, S., Van Caneghem, J., 2020. Closing the phosphorus cycle: Multi-criteria techno-economic optimisation of phosphorus extraction from wastewater treatment sludge ash. Science of the Total Environment. 713, 135543. https://doi.org/10.1016/j.scitotenv.2019.135543
Acknowledgements
This work was supported by Fonds Wetenschappelijk Onderzoek Vlaanderen (FWO) (Belgium) [project number 1S08418N]. We want to thank Aquafin for supplying the WWT sludge ash sample. Furthermore, we want to thank Antoinette Deschuytere for her input on the explanation of the experimental data by means of Ksp's and chemical equilibriums.
Biography lead author: Lorien Luyckx
 Lorien Luyckx graduated in June 2016 as a Master in Chemical Engineering Technology. In October 2016, she started her PhD on phosphorus recovery from incineration ash of phosphorus rich waste streams under supervision of prof. Jo Van Caneghem (ChEMaRTS). During her PhD, she will work on phosphorus extraction and phosphorus recovery in fertilizers or other applications. Her PhD will also include a study examining the influence of combustion parameters on the phosphorus mineralogy of incineration ash (thermodynamic simulations), Life Cycle Analysis and Techno-Economic Assessment.
Lorien Luyckx graduated in June 2016 as a Master in Chemical Engineering Technology. In October 2016, she started her PhD on phosphorus recovery from incineration ash of phosphorus rich waste streams under supervision of prof. Jo Van Caneghem (ChEMaRTS). During her PhD, she will work on phosphorus extraction and phosphorus recovery in fertilizers or other applications. Her PhD will also include a study examining the influence of combustion parameters on the phosphorus mineralogy of incineration ash (thermodynamic simulations), Life Cycle Analysis and Techno-Economic Assessment.