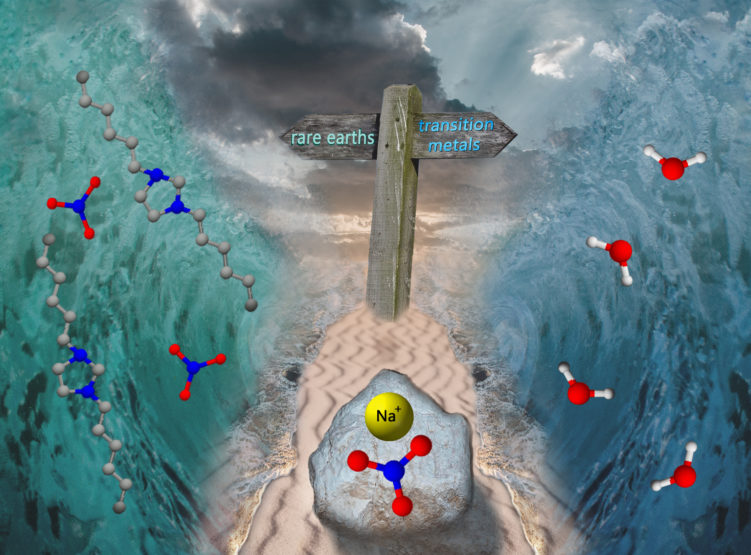
Daphne Depuydt and Arne Van den Bossche from the group of prof. Koen Binnemans recently published a Communication on “Metal extraction with a short-chain imidazolium nitrate ionic liquid”. As the article was well received by the reviewers and the editorial office of the Chemical Communications journal , it was rewarded with an inside cover. The idea behind the graphical artwork, designed by dr. Joris Roosen, comes from the story of the Old Testament, where Moses splits the sea to guide his people. The cover art shows NaNO3 splitting the sea, with rare earths travelling towards the ionic liquid sea and transition metals moving to the sea filled with water.
Under investigation in the work of Depuydt and Van den Bossche is the 1,3-dihexylimidazolium nitrate ionic liquid-water system which shows upper critical solution temperature (UCST) phase behavior. This means that above the critical temperature, the system becomes a fully homogeneous mixture. The concept of the thermomorphic ionic liquids is not new, yet a novel system with a simple, lowly viscous, short-chain imidazolium ionic liquid was developed. Normally, thermomorphic behavior is exploited in homogeneous liquid-liquid extraction studies. Yet, the addition of salts, in this case sodium nitrate (NaNO3), led to the disappearance of the temperature-dependent miscibility behavior. Instead, a fully biphasic system was found at all temperatures, leading to a hydrophobic ionic liquid system with only half the number of carbons than the usual hydrophobic ionic liquids used in metal extraction studies. The short-chain ionic liquid was used for the separation of rare earths from transition metals.
The article is open access and can be downloaded here. Full reference: Daphne Depuydt, Arne Van den Bossche, Wim Dehaen and Koen Binnemans, Metal extraction with a short-chain imidazolium
nitrate ionic liquid, Chemical Communications, 53 (38), 2017, 5271-5274.
Inside Cover Chemical Communications