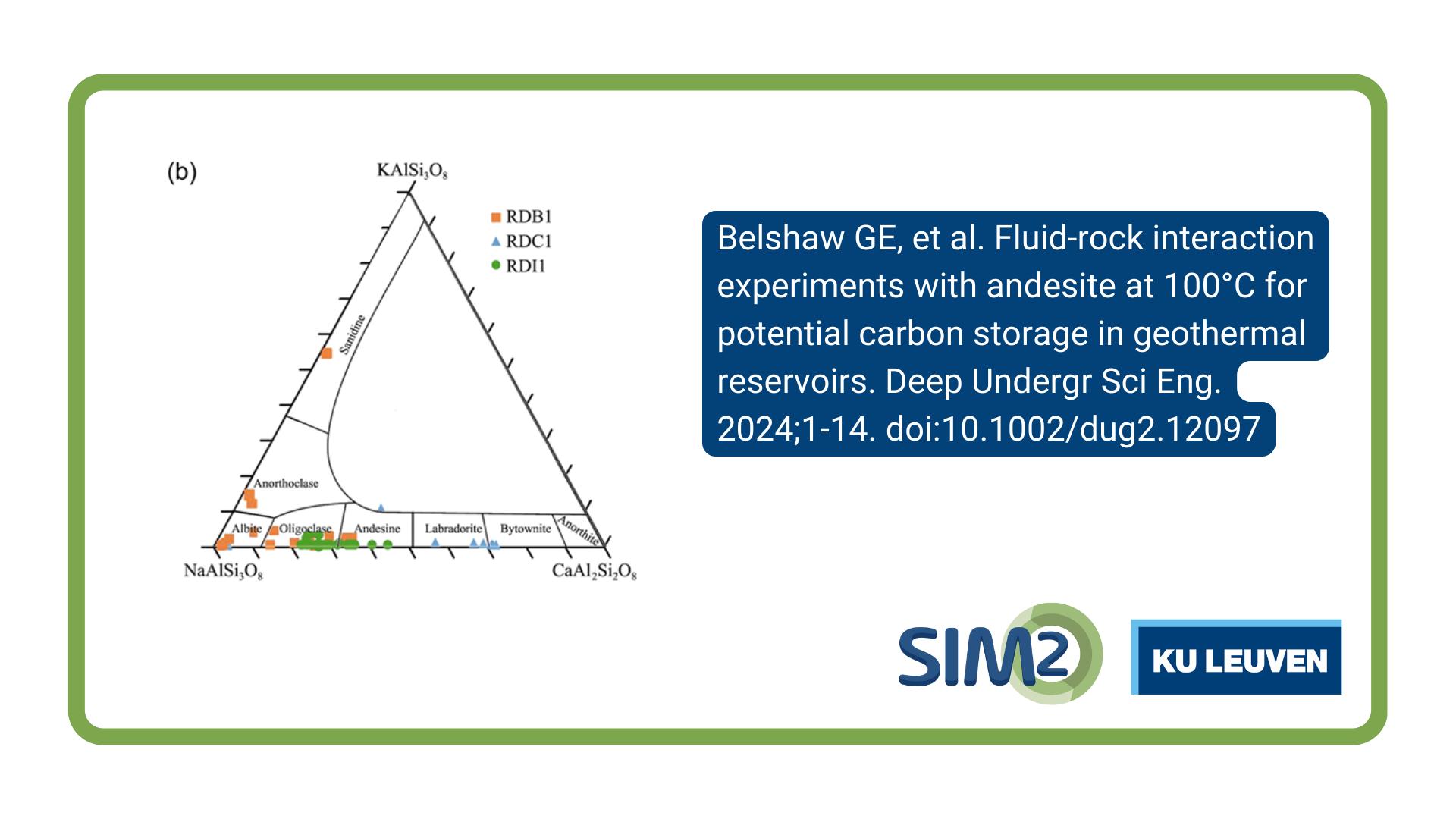
Veerle Vandeginste from the Department of Materials Engineering, KU Leuven, together with colleagues from the UK, China and Indonesia, has published an article entitled Fluid‐rock interaction experiments with andesite at 100°C for potential carbon storage in geothermal reservoirs in the journal Deep Underground Science and Engineering.
Geothermal energy extraction often results in the release of naturally occurring carbon dioxide (CO2) as a byproduct. Research on carbon storage using volcanic rock types other than basalt under both acidic and elevated temperature conditions has been limited so far. Our study uses batch reactor experiments at 100°C to investigate the dissolution of andesite rock samples obtained from an active geothermal reservoir in Sumatra (Indonesia). The samples are subjected to reactions with neutral‐pH fluids and acidic fluids, mimicking the geochemical responses upon reinjection of geothermal fluids, either without or with dissolved acidic gases, respectively. Chemical elemental analysis reveals the release of Ca2+ ions into the fluids through the dissolution of feldspar. The overall dissolution rate of the rock samples is 2.4×10–11mol/(m2·s) to 4.2×10–11mol/(m2·s), based on the Si release during the initial 7 h of the experiment. The dissolution rates are about two orders of magnitude lower than those reported for basaltic rocks under similar reaction conditions. This study offers valuable insights into the potential utilization of andesite reservoirs for effective CO2 storage via mineralization.
Reference
Belshaw GE, Steer E, Ji Y, et al. Fluid‐rock interaction experiments with andesite at 100°C for potential carbon storage in geothermal reservoirs. Deep Undergr Sci Eng. 2024;1‐14. doi:10.1002/dug2.12097
Acknowledgement
The authors thank Mark Guyler for training and maintenance of the ICP‐OES equipment and systems and practical advice on experimental setup, William Lewis and Stephen Argent for training and maintenance of the PXRD equipment, and Aleksandra Gonciaruk for training and maintenance of the 3flex sorption equipment. This work was supported by the Engineering and Physical Sciences Research Council (EPSRC) Strategic Development Grant for the Gas Adsorption Analysis Suite [under grant EP/M000567/1], the GeoEnergy Research Centre (GERC) Nottingham Strategic Development Fund, and the University of Nottingham. The authors thank the Nanoscale and Microscale Research Centre (nmRC) for providing access to the Microprobe instrumentation. The authors thank Teresa Needham and the School of Geography for access to XRF analysis. Alfend Rudyawan and Marino Baroek are warmly acknowledged for their continued support and fruitful discussions. Thanks are due to the Royal Society of Chemistry and the Geochemistry Group of the Geological Society, who provided travel grants for the first author to present this work at Goldschmidt 2019.