SIM² KU Leuven – HiTemp researchers studied the characterisation of antimony-containing metallurgical residues in view of enhanced antimony recovery. Based on the obtained characterisation results, feasible Sb recovery technologies and their potential challenges are evaluated. The results were published in the Journal of Cleaner Production.
Why antimony?
Antimony is a very versatile element possessing a combination of metal and non-metal properties. Antimony metal is mainly used as an alloying component in lead applications, including lead-acid batteries. Antimony trioxide (ATO) is the most significant antimony compound produced, mainly as a synergist for halogenated flame retardants and the catalysis of PET resin. Antimony has been on the EU list of Critical Raw Materials because Europe strongly relies on imports, thereby increasing the supply risk. Therefore, Sb recovery from metallurgical residues is an important route to mitigate Europe’s Sb supply risk.
Characterisation of Sb-containing metallurgical residues
This paper reports a complete characterisation of three typical Sb-containing metallurgical residues, namely crude antimony trioxide (CATO), lead softening slag (LSS), and antimony slag (AS). CATO contains arsenic as the main impurity. LSS is rich in lead and contains antimony and trace arsenic. AS is rich in antimony with a fraction of Al, Si, Fe, Pb, et al.
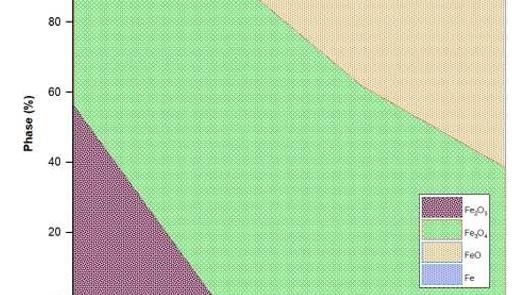
Antimony is present as Sb2O3 in CATO and AS, while as complex oxides like Pb2(As, Sb)2O7 and Pb3+x(Sb, As)2O8+x in LSS. Arsenic oxide and antimony oxide form solid solution in both CATO and AS. TG-DSC analysis indicates Sb2O3 volatilises in the nitrogen atmosphere but undergoes oxidation to Sb2O4 in the air with increasing the temperature up to 1000 °C.
Feasible Sb recovery technologies and potential challenges
Carbothermic reduction method is feasible for the Sb recovery from CATO and AS. The challenge, howver, is the selection of the slag system. The slag fuming method is applicable to produce ATO from CATO and AS. The removal of arsenic from CATO is essential. The slag composition and kinetic parameters play a critical role in the AS fuming process. Arsenic could be removed from LSS using sodium hydroxide roasting followed by water leaching.
The amount of NaOH added is the crucial parameter. The As-depleted LSS can be subjected to a carbothermic reduction process to recover Pb and Sb. Sb can be recycled in the form of ATO or crude antimony, depending on the reductant type and dosage.
Full reference of paper
Hongbin Ling, Bart Blanpain, Muxing Guo, Annelies Malfliet (2021), Characterization of antimony-containing metallurgical residues for antimony recovery, Journal of Cleaner Production, 327, 129491, https://doi.org/10.1016/j.jclepro.2021.129491.
Acknowledgments
The authors would like to thank Campine for providing the materials used in this study. Hongbin Ling acknowledges the support of the KU Leuven HiTemp Centre and the China Scholarship Council (CSC, Grant No. 201806370218).