A team of SIM² KU Leuven/SOLVOMET & ArcelorMittal researchers has developed an ammoniacal leaching process to valorise Zn-rich BOF sludges from the steel industry. The work, which was published as a Golden Open Access paper in the Journal of Sustainable Metallurgy, was performed in the framework of the EIT RawMaterials SAMEX project, in which this ammoniacal extraction process is being upscaled by Tecnalia, ArcelorMittal and KU Leuven.
Zn-rich BOF sludges
During the production of steel large volumes of steel by-products are generated, ranging from slags, dusts and sludges, which are produced blast furnaces (BFs), electric arc furnaces (EAFs) and basic oxygen furnaces (BOFs).
One of the most challenging steel by-products is the BOF sludge. The zinc content of basic oxygen furnace (BOF) sludges is too high for direct recycling into the blast furnace via the sinter plant, as excessive zinc concentrations are detrimental for the refractory lining of the blast furnace.
However, by partial and selective removal of zinc from the BOF sludge, the residual sludge can be used as a secondary iron resource in the blast furnace.
Ammoniacal leaching of Zn-rich BOF sludge (Download paper here)
To support the potential (internal) recycling of Zn-rich BOF sludges, the present SOLVOMET KU Leuven/ArcelorMittal research team developed a process that selectively removes Zn from the BOF sludges, leaving behind a Zn-depleted, Fe-rich residue that can be sent back to the blast furnace, via the sinter plant.
In this new paper, BOF sludge was, therefore, leached with aqueous ammonia, aqueous solutions of ammonium salts (chloride, carbonate, and sulphate), and aqueous mixtures of ammonia and ammonium salt. The mixtures of ammonia and ammonium salt could leach more zinc with respect to either the aqueous ammonia or the aqueous ammonium salt solution.
AAC leaching most effective
The ammonia–ammonium carbonate (AAC) mixture was selected as the most suitable lixiviant due to the high zinc leaching efficiency in combination with a high selectivity towards iron; furthermore, this combination does not introduce unwanted chloride or sulphate impurities in the residue.
The leaching process was optimised in terms of the liquid-to-solid ratio, total ammonia concentration, ammonium:ammonia molar ratio, temperature, and leaching time. The co-dissolved iron was precipitated as a hydroxide after oxidation of ferrous to ferric ions by an air stream, without co-precipitation of zinc, while the dissolved zinc could be easily recovered as zinc sulphide by precipitation with ammonium sulphide. The (almost) closed-loop process was successfully up-scaled from 10 mL to 1 L scale.
Franklinite
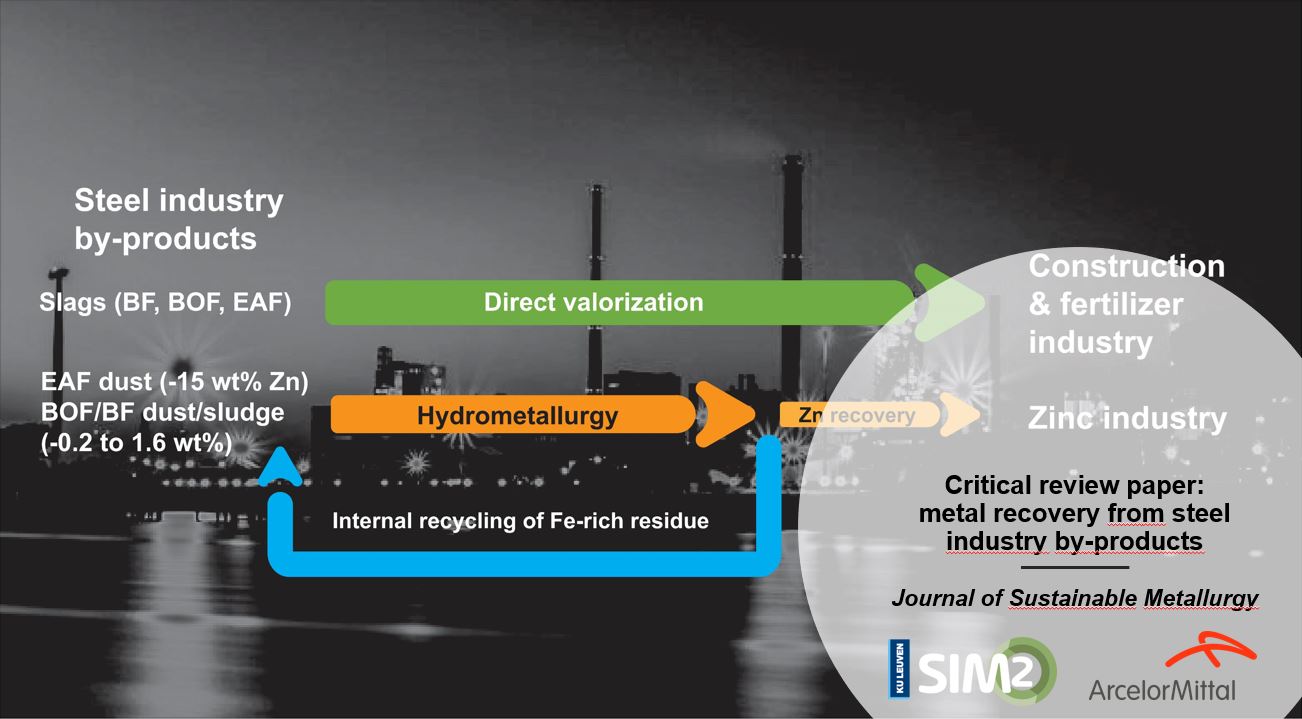
As also described in a parallel SAMEX-derived publication – a 35-page review paper on the hydrometallurgical recovery of metals from distinct steelmaking by-products – the leaching of franklinite is not impossible but requires much harsher leaching conditions, which come with their own operational problems.
Full reference of paper
Nerea Rodriguez Rodriguez, Lukas Gijsemans, Jakob Bussé, Joris Roosen, Mehmet Ali Recai Önal, Victoria Masaguer Torres, Álvaro Manjón Fernández, Peter Tom Jones, · Koen Binnemans, Selective Removal of Zinc from BOF Sludge by Leaching with Mixtures of Ammonia and Ammonium Carbonate, Journal of Sustainable Metallurgy, 2020. [Download Open Access paper here]
Acknowledgements
The authors acknowledge funding from the European Institute of Innovation and Technology (EIT), a body of the European Union, under Horizon 2020, part of the ‘KAVA Call 6,’ in the framework of the ’Innovation Theme’ No.3 of EIT Raw Materials Project Number 19205 (SAMEX). Nerea Rodriguez Rodriguez acknowledges the financial support from the Research Foundation‐Flanders (FWO, Grant nr. 12X5119N, postdoctoral fellowship).
More info about SAMEX
For each mtonne of steel ArcelorMittal (AM) produces, it also generates on average 10,000 t of Zn-rich, fine Basic Oxygen Furnace (BOF) steelmaking sludge. In contrast with the coarse BOF sludge fraction, which is already internally recycled by ArcelorMittal, the fine BOF sludge fraction cannot be fed to the Blast Furnace (BF), as the Zn content would lead to prohibitive refractory failure and disturbances in the BF process. As a result, ArcelorMittal either internally stores these sludges or is forced to landfill them.
To avoid excessive landfilling and to create an industrial symbiosis system, ArcelorMittal developed in 2017-2018, in collaboration with SOLVOMET KU Leuven, an ammoniacal leaching process. The developed process selectively extracts Zn from the sludge (obtaining a 76% leaching yield) while leaving behind most iron. The cleaned, Fe-rich residue can be fed to the BF, via the sinter plant, representing major iron cost savings. Concurrently, the leached Zn in the pregnant leach solution can be recovered as a ZnS-precipitate product, as a feed for the zinc industry.
In the SAMEX project, Tecnalia (Spain), ArcelorMittal (Spain) and KU Leuven (Belgium) shall upscale the ammoniacal leaching process to TRL7, aiming to engineer and build a pilot plant. The pilot plant will be used to demonstrate and validate the flowsheet, using distinct BOF sludges from different ArcelorMittal plants in Europe. If successful, ArcelorMittal foresees to implement the process in at least one third of its EU-plants by 2025 (i.e. treatment of 120,000 t/year BOF fine sludge). Furthermore, other sludge producers and steelmaking companies will be able to benefit from the results generated in the project.
Website: https://eit-samex.eu/