Prof. Tom Van Gerven is Head of the Process Engineering for Sustainable Systems section in the Department of Chemical Engineering at KU Leuven. Within the KU Leuven Institute for Sustainable Metals and Minerals (SIM²), he has been the driving force behind Research Line 6 on Process Intensification and Digitalisation. During the past years he has been implementing these principles in metallurgical processes, including solvent extraction. In the present interview you get to know more about Van Gerven’s research activities and philosophy.
During the past years the European Commission has expressed its ambition to make the EU climate neutral by 2050. As we all know, the metallurgical industry, and in particular the steel industry, is highly energy- and CO2-intensive. How is your research on process intensification aligned with these developments?
With process intensification we study the engineering surrounding the chemical reaction itself. This means we are looking to reduce transfer limitations of mass, heat and momentum (mixing), and find better ways of activating chemical reactions. We develop reactor and separation systems that are more efficient in terms of material or energy usage.
Industrially successful examples of process intensification include microreactors (typically of interest for pharmaceutical applications) and reactive distillation (for bulk chemistry). Process intensification can be performed via a number of approaches. The approach that I am taking is the use of alternative energy sources, such as ultrasound and light.
Ultrasound allows to mix on a microscale, unlike stirring, thus improving mass transfer near interfaces, which can for example yield quicker reactions [see papers John et al. below].
With light we are looking at the development of photochemical reactors where the light rays are used as efficiently as possible without too much dissipation [see papers Van den Bogaert et al, Leblebici et al, Claes et al below]. The bottom line is that we try to make reactors and separators, the workhorses in a chemical plant, more energy- and material efficient.
.jpg)
Image: Tom Van Gerven is holding an example of a custom-made milli-flow reactor with an off-the-shelf ultrasound transducer attached to it.
You mainly refer to the chemical engineering industry. However, you have multiple projects in the domain of developing new reactor types for the solvent extraction of metals. In which direction do you see this moving? What are the challenges?
The solvent extraction research line is something that I am developing in close collaboration with Prof. Koen Binnemans (SOLVOMET Group, Department of Chemistry). Koen investigates new chemistries, while we apply these chemistries in novel reactors.
We particularly look out for the really difficult cases, for example highly viscous phases where interfacial mass transfer is difficult and slow. Most of our efforts until now have been devoted to (ultrasound-assisted) microreactors, but because of the small dimensions, their productivity is rather low. Although the scale up of microreactors or other types of reactors is challenging, it is crucially important for the materials sector.
.jpg)
Image: The experimental set-up for continuous-flow solvent extraction
In the European project SIMPLIFY [Sonication and Microwave Processing of Material Feedstock] we are now also looking at the use of ultrasound in oscillating baffle flow reactors for the production of zeolites [see paper Mendoza] and in twin screw extruders for the production of polymers. Throughputs can be easily in the order of 10,000s of tonnes of material per year. Furthermore, with the clever positioning of ultrasound activation where it is needed, substantial rate improvements can be achieved. This should also be possible in the case of solvent extraction, which we are currently investigating in a Flemish-funded project (FWO ISOMER).
In this project we focus on the use of ionic liquids for the extraction of critical metals from aqueous liquids in microreactors. The main engineering challenge is the high viscosity of undiluted ionic liquids, which hinders the formation of vortices in the two-phase slug flow, thus slowing down transfer of the metals over the organic-aqueous interface. We are now characterising the mass transfer efficiency to understand how selected processing conditions and reactor geometries can maximize this efficiency without giving in on the selection of the ionic liquid.
The European Commission has recently published its fourth list of Critical Raw Materials (CRM) and has launched the somewhat Orwellian term of “open strategic autonomy”. In its new list, lithium has now been officially identified as a “Critical Raw Material”. You have been performing some work on lithium extraction and refining. How can process intensification help here?
That’s right: some of the technologies investigated in my group may also be applied in the case of lithium. Solvent extraction was already mentioned. Another potential area that I see is that of selective crystallisation [see paper Jordens et al below] where you obtain a lithium salt at lower dissolved concentrations (more diluted streams) and with higher purity (more selective).
With the application of, again, ultrasound, we’ve shown that the lithium recovery rate can reach 80% with a lithium concentration of 10 g/L, while the obtained Li2CO3 purity level is of industrial grade, by a one-step precipitation [see paper Zhao et al below].
Over the past years you have published numerous peer-reviewed papers. Which paper of yours are you particularly pleased with?
The latest paper is always the best one! On a more serious note, I’m quite satisfied with the fact that there is a healthy distribution between more fundamental and more applied papers, between novel concepts and further development, between research and review papers.
Do you have any advice for PhD students in terms of publishing papers?
Start with the message you want to convey. Often it helps to first make the most important graph of your future paper, and then build your story around that graph.
What attracts you in research? How do you stay motivated?
The most attractive part in research for me is the moment of writing a research proposal. At that moment, everything is still possible and you can really shape the proposal around topics of your interest.
You formulate a hypothesis supported by theoretical considerations, and then slice up the path to (dis)prove this hypothesis into research tasks. Once the project is granted, it is like a child that you hand over to your PhD students. I’m obviously still interested in the further developments but it never reaches the intimate feeling again that you had when conceiving the proposal.
My motivation also comes from the almost total freedom I have in defining the research area I want to be in. In 2012, for example, I got interested in games and virtual/augmented reality. I started writing a small research project to apply these immersive tools to chemical engineering education, and now, less than 10 years later, I am coordinating a European Training Network on Immersive Learning for Chemical Engineering (ETN CHARMING).
Finally, just for our readers to know, what is the strangest talent you have?
I am world famous in my family for my stacking skills. I can make extremely stable piles of bags and other stuff in our car when we go on holiday. If anybody needs help, I am always available.
Thank you for this interview. We wish you all the best in your research endeavours.
[Credits: Interview: Peter Tom Jones – Photographs: Elisabeth De Decker – Interviews with other SIM² colleagues: https://kuleuven.sim2.be/meet-our-colleagues/]
Biography Prof Tom Van Gerven
 Prof. Tom Van Gerven is Head of the Process Engineering for Sustainable Systems section (50 people) in the Department of Chemical Engineering at KU Leuven. He is currently (co-)promoter of 1 Postdoc and 19 PhD students, and has promoted 18 finalised PhDs. He is the author of 175 peer-reviewed publications and has an h-index of 41. He currently coordinates 3 European projects. He is chairman of the EFCE Working Party on Process Intensification and serves on several boards (e.g., European Society of Sonochemistry, Expert panel Chemical Engineering and Materials Science of FWO, Journal of Advanced Manufacturing and Processing). He is a Steering Committee member of the KU Leuven Institute for Sustainable Metals and Minerals (SIM²), in which he is one of the driving forces of Research Line 6 on Process Intensification and Digitalisation.
Prof. Tom Van Gerven is Head of the Process Engineering for Sustainable Systems section (50 people) in the Department of Chemical Engineering at KU Leuven. He is currently (co-)promoter of 1 Postdoc and 19 PhD students, and has promoted 18 finalised PhDs. He is the author of 175 peer-reviewed publications and has an h-index of 41. He currently coordinates 3 European projects. He is chairman of the EFCE Working Party on Process Intensification and serves on several boards (e.g., European Society of Sonochemistry, Expert panel Chemical Engineering and Materials Science of FWO, Journal of Advanced Manufacturing and Processing). He is a Steering Committee member of the KU Leuven Institute for Sustainable Metals and Minerals (SIM²), in which he is one of the driving forces of Research Line 6 on Process Intensification and Digitalisation.
Some key papers by Prof. Tom Van Gerven in the field of (metallurgical) process intensification
- Santos, R., Ceulemans, P., Van Gerven, T. (2012). Synthesis of pure aragonite by sonochemical mineral carbonation. Chemical Engineering Research & Design, 90 (6), 715-725. doi: 10.1016/j.cherd.2011.11.022 Open Access
- Borra, C.R., Pontikes, Y., Binnemans, K., Van Gerven, T. (2015). Leaching of rare earths from bauxite residue (red mud). Minerals Engineering, 76, 20-27. doi: 10.1016/j.mineng.2015.01.005 Open Access
- Van den Bogaert, B., Havaux, D., Binnemans, K., Van Gerven, T. (2015). Photochemical recycling of europium from Eu/Y mixtures in red lamp phosphor waste streams. Green Chemistry, 17 (4), 2180-2187. doi: 10.1039/C4GC02140A Open Access
- Jordens, J., De Coker, N., Gielen, B., Van Gerven, T., Braeken, L. (2015). Ultrasound precipitation of manganese carbonate: the effect of power and frequency on particle properties. Ultrasonics Sonochemistry, 26, 64-72. doi: 10.1016/j.ultsonch.2015.01.017 Open Access
- Beyene, E.M., Smets, I., Van Gerven, T. (2016). Ultrasound assisted chrome tanning: Towards a clean leather production technology. Ultrasonics Sonochemistry, 32, 204-2012.
- Van Loy, S., Binnemans, K., Van Gerven, T. (2017). Recycling of rare earths from lamp phosphor waste: Enhanced dissolution of LaPO4:Ce3 ,Tb3 by mechanical activation. Journal of Cleaner Production, 156, 226-234. doi: 10.1016/j.jclepro.2017.03.160
- Leblebici, M.E., Van den Bogaert, B., Stefanidis, G., Van Gerven, T. (2017). Efficiency vs. productivity in photoreactors, a case study on photochemical separation of Eu. Chemical Engineering Journal, 310 (1), 240-248. doi: 10.1016/j.cej.2016.10.112
- John, J.J., Kuhn, S., Braeken, L., Van Gerven, T. (2018). Effect of fluid properties on ultrasound assisted liquid-liquid extraction in a microchannel. Ultrasonics Sonochemistry, 42, 68-75.
- Zhao, C., Zhang, Y., Cao, H., Zheng, X., Van Gerven, T., Hu, Y., Sun, Z. (2019). Lithium carbonate recovery from lithium-containing solution by ultrasound assisted precipitation. Ultrasonics Sonochemistry, 52, 484-492. doi: 10.1016/j.ultsonch.2018.12.025
- Jordens, J., Gielen, B., Xiouras, C., Hussain, M., Stefanidis, G., Thomassen, L., Braeken, L., Van Gerven, T. (2019). Sonocrystallisation: Observations, theories and guidelines. Chemical Engineering and Processing: Process Intensification, 139, 130-154. doi: 10.1016/j.cep.2019.03.017
- Claes, T., Dilissen, A., Leblebici, M.E., Van Gerven, T. (2019). Translucent packed bed structures for high throughput photocatalytic reactors. Chemical Engineering Journal, 361, 725-735. doi: 10.1016/j.cej.2018.12.107
- Mendoza, H.R., Jordens, J., Pereira, M.V L., Lutz, C., Van Gerven, T. (2020). Effects of ultrasonic irradiation on crystallization kinetics, morphological and structural properties of zeolite FAU. Ultrasonics Sonochemistry, 64, Art.No. ARTN 105010. doi: 10.1016/j.ultsonch.2020.105010
Want to know more about SIM²?
SIM² KU Leuven is the KU Leuven Institute for Sustainable Metals and Minerals (SIM² in short). SIM² is one of the five official KU Leuven Institutes that haven been endorsed by the KU Leuven Academic Council. SIM² has more than 240 members, coming from a wide range of (interdisciplinary) research groups and departments at KU Leuven.
SIM²’s missions is “to develop, organise & implement problem-driven, science-deep research & future-oriented education, contributing to the environmentally friendly production & recycling of metals, minerals & engineered materials, supporting (…) a climate-friendly, circular-economy”. Read more about the new KU Leuven Institute here.
Follow SIM² on LinkedIn: https://www.linkedin.com/company/18118889
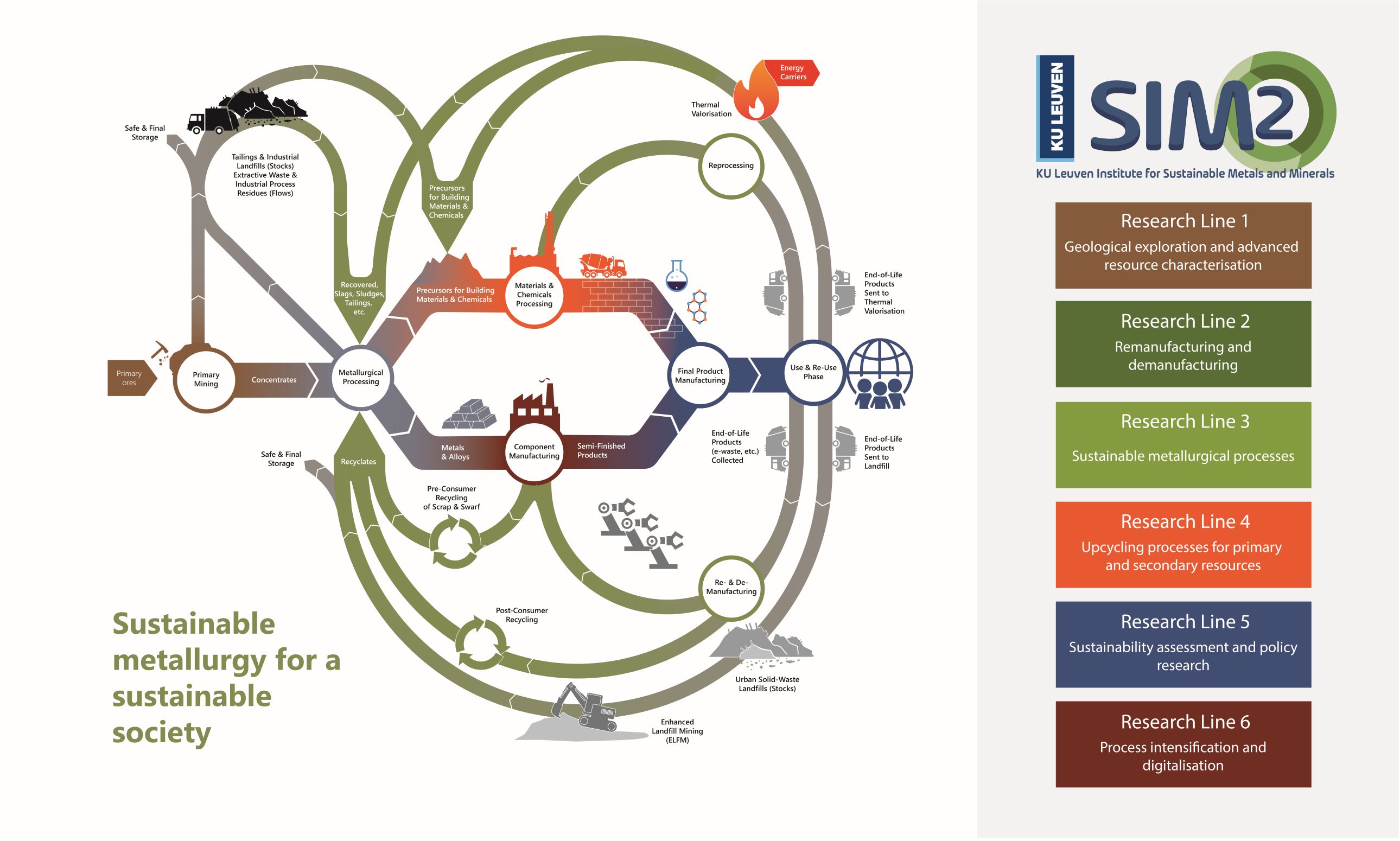
Image: SIM² KU Leuven's view on the Circular Economy and connections with Research Lines